Akkattu T. Biju
Associate Professor
Phone : +91-80-2293-2646(O)
E-mail: atbiju@iisc.ac.in
Home Page : http://atbiju.in/
Biography
Education
- 2002-2007: Ph. D., Organic Chemistry Division, CSIR-NIIST, Trivandrum, Kerala, mentor: Dr. G. Vijay Nair
- 1999-2001: M. Sc. Chemistry, MG University, Kottayam, Kerala.
- 1995-1998: B. Sc. Chemistry (Petrochemicals), MG University, Kottayam, Kerala.
Professional Experience
- June 2017- Present: Associate Professor, Department of Organic Chemistry, IISc Bangalore
- June 2011- May 2017: Senior Scientist, Organic Chemistry Division, CSIR-NCL, Pune
- 2008-2011: Alexander von Humboldt Post-doctoral fellow, University of Münster, mentor: Dr. Frank Glorius
- 2007-2008: Post-doctoral fellow, National Taiwan University, Taiwan, mentor: Tien-Yau Luh
Research Interests
Transition-Metal-Free Carbon-Carbon and Carbon-Heteroatom Bond-Forming Reactions and their Application in Organic Synthesis
Aryne Chemistry
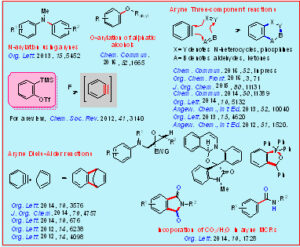
 Arynes are highly electrophilic intermediates, which are widely used for the synthesis of various benzo-fused carbocycles and heterocycles of structural complexity and diversity. In the area of aryne chemistry, we have recently developed mild, efficient and scalable Diels-Alder reaction of arynes with challenging diene systems such as pentafulvenes, 1,2-benzoquinones, styrenes, indenes/ benzofurans, and tropones. Moreover, the synthetic utility of N-heterocycles such as aziridines, pyridines, and (iso)quinolines in aryne multicomponent reactions (MCRs) has been demonstrated for the synthesis of various heterocycles. In addition, we have developed aryne MCRs triggered by phosphines for the synthesis of functionalized benzooxaphospholes, and the use of CO2 as a one-carbon synthon in aryne MCRs has been developed. Furthermore, a transition-metal-free protocol for the N-arylation of tertiary amines as well as the O-arylation of aliphatic alcohols has been developed.
Arynes are highly electrophilic intermediates, which are widely used for the synthesis of various benzo-fused carbocycles and heterocycles of structural complexity and diversity. In the area of aryne chemistry, we have recently developed mild, efficient and scalable Diels-Alder reaction of arynes with challenging diene systems such as pentafulvenes, 1,2-benzoquinones, styrenes, indenes/ benzofurans, and tropones. Moreover, the synthetic utility of N-heterocycles such as aziridines, pyridines, and (iso)quinolines in aryne multicomponent reactions (MCRs) has been demonstrated for the synthesis of various heterocycles. In addition, we have developed aryne MCRs triggered by phosphines for the synthesis of functionalized benzooxaphospholes, and the use of CO2 as a one-carbon synthon in aryne MCRs has been developed. Furthermore, a transition-metal-free protocol for the N-arylation of tertiary amines as well as the O-arylation of aliphatic alcohols has been developed.
NHC Organocatalysis
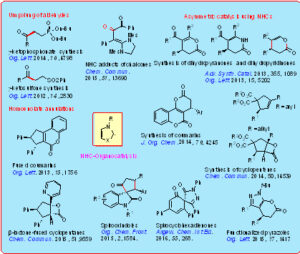
 In another phase of the work using NHC-catalysis, we have developed efficient and facile Stetter reaction using vinyl sulfones, and vinyl phosphonates as Michael acceptors. Moreover, in the area of homoenolate annulations using NHCs, we have developed efficient methods for the diastereoselective synthesis of cyclopentane-fused coumarins, cyclopentane-fused b-lactones and spirocyclopentanone oxindoles. We are also working on asymmetric catalysis using NHCs. We have recently developed a facile method for the enantioselective synthesis of functionalized dihydropyranones and dihydropyridinones by the reaction of modified enals with b-dicarbonyl compounds or enaminones, enolizable aldehydes, heterocyclic C-H acids, and pyrazolones. Furthermore, an enantioselective synthesis of functionalized cyclopentenes, and b-lactone-fused cyclopentanes by the NHC-catalyzed reaction of 2-bromoenals with a-substituted b-diesters has been uncovered. Very recently, we have also demonstrated the enantioselective synthesis of pyrazolone-fused spirocyclohexadienones by the reaction of a,b-unsaturated aldehydes with a-arylidene pyrazolinones under oxidative NHC-catalysis.
In another phase of the work using NHC-catalysis, we have developed efficient and facile Stetter reaction using vinyl sulfones, and vinyl phosphonates as Michael acceptors. Moreover, in the area of homoenolate annulations using NHCs, we have developed efficient methods for the diastereoselective synthesis of cyclopentane-fused coumarins, cyclopentane-fused b-lactones and spirocyclopentanone oxindoles. We are also working on asymmetric catalysis using NHCs. We have recently developed a facile method for the enantioselective synthesis of functionalized dihydropyranones and dihydropyridinones by the reaction of modified enals with b-dicarbonyl compounds or enaminones, enolizable aldehydes, heterocyclic C-H acids, and pyrazolones. Furthermore, an enantioselective synthesis of functionalized cyclopentenes, and b-lactone-fused cyclopentanes by the NHC-catalyzed reaction of 2-bromoenals with a-substituted b-diesters has been uncovered. Very recently, we have also demonstrated the enantioselective synthesis of pyrazolone-fused spirocyclohexadienones by the reaction of a,b-unsaturated aldehydes with a-arylidene pyrazolinones under oxidative NHC-catalysis.
Representative Publications
- Uncovering the Neglected Similarities of Arynes and Donor-Acceptor Cyclopropanes. Werz, D. B.; Biju, A. T. Angew. Chem. Int. Ed. 2020, 59, 3385.
- N-Heterocyclic Carbene-Catalyzed Umpolung of Imines for the Enantioselective Synthesis of Dihydroquinoxalines. Das, T. K.; Ghosh, A.; Balanna, K.; Behera, P.; Gonnade, R. G.; Marelli, U. K.; Das, A. K.; Biju, A. T. ACS Catal. 2019, 9, 4065.
- NHC-Catalyzed Generation of α,β-Unsaturated Acylazoliums for the Enantioselective Synthesis of Heterocycles and Carbocycles. Mondal, S.; Yetra, S. R.; Mukherjee, S.; Biju, A. T. Acc. Chem. Res. 2019, 52, 425.
- N-Heterocyclic Carbene-Catalyzed Aldol-Lactonization of Ketoacids via Dynamic Kinetic Resolution. Mondal, S.; Mukherjee, S.; Das, T. K.; Gonnade, R. G.; Biju, A. T. ACS Catal. 2017, 7, 3995.
- N-Heterocyclic Carbene-Catalyzed Umpolung of Imines, Patra, A.; Mukherjee, S.; Das, T. K.; Jain, S.; Gonnade, R. G.; Biju, A. T. Angew. Chem. Int. Ed. 2017, 56, 2730.
- Employing Arynes in Transition-Metal-Free Carbon-Carbon and Carbon-Heteroatom Bond-Forming Reactions. Bhojgude, S. S.; Bhunia, A.; Biju, A. T. Acc. Chem. Res. 2016, 49, 1658.
- Lewis Acid-Catalyzed Selective Reactions of Donor-Acceptor Cyclopropanes with 2-Naphthols. Kaicharla, T.; Roy, T.; Thangaraj, M.; Gonnade, R. G.; Biju, A. T. Angew. Chem. Int. Ed. 2016, 55, 10061.
- Enantioselective Synthesis of Spirocyclohexadienones by NHC-Catalyzed Formal [3+3] Annulation Reaction of Enals. Yetra, S. R.; Mondal, S.; Mukherjee, S.; Gonnade. R. G.; Biju, A. T. Angew. Chem. Int. Ed. 2016, 55, 268.
- Transition-Metal-Free Multicomponent Reactions Involving Arynes, N-Heterocycles and Isatins. Bhunia, A.; Roy, T.; Pachfule, P.; Rajamohanan, P. R.; Biju, A. T. Angew. Chem., Int. Ed. 2013, 52, 10040.
- Recent Advances in Transition-Metal-Free Carbon-Carbon and Carbon-Heteroatom Bond-Forming Reactions Using Arynes. Bhunia, A.; Yetra, S. R.; Biju, A. T. Chem. Soc. Rev. 2012, 41, 3140.
- Arynes in Transition-Metal-Free Multicomponent Coupling Reactions. Bhojgude, S. S.; Biju, A. T. Angew. Chem., Int. Ed. 2012, 51, 1520.
Awards / Honours / Affiliations
- Fellow of Royal Society of Chemistry- FRSC (2019)
- CRSI Bronze Medal, Chemical Research Society of India (2019)
- Editor-in-Chief, J. Heterocyclic Chem. (Wiley)
- Bhagyatara Award by Panjab University (2018)
- SERB Distinguished Investigator Award (2018)
- AVRA Young Scientist Award (2016) [A V Rama Rao Research Foundation]
- CRSI Young Scientist Award (2015) [Chemical Research Society of India]
- NCL-RF Scientist of the Year Award (2014) [National Chemical Laboratory Research Foundation]
- ISCB Young Scientist Award (2014) [Indian Society for Chemists and Biologists]
- Thieme Chemistry Journals Award (2014)
- OPPI Young Scientist Award (2012) [Organization of Pharmaceutical Producers of India]
- Alexander von Humboldt post-doctoral fellowship (2009-2010)
- Fellowship of the National Science Council, Taiwan (2007)
- CSIR Junior Research Fellowship (2001)
- University First Rank M.Sc. Chemistry, MG University, Kerala (2001)
- University First Rank B.Sc. Petrochemicals, MG University, Kerala (1998)


